Compressed gases are commonly used in lab and present a number of hazards for the laboratory worker.
- Gas cylinders may contain gases that are flammable, toxic, corrosive, asphyxiants, or oxidizers.
- Unsecured cylinders can be easily knocked over, causing serious injury and damage.
- Impact can shear the valve from an uncapped cylinder, causing a catastrophic release of pressure leading to personal injury and extensive damage.
- Mechanical failure of the cylinder, cylinder valve, or regulator can result in rapid diffusion of the pressurized contents of the cylinder into the atmosphere; leading to explosion, fire, runaway reactions, or burst reaction vessels.
Handling Compressed Gas Cylinders
The contents of any compressed gas cylinder must be clearly identified. Identification should be stenciled or stamped on the cylinder, or a label or tag should be attached. Do not rely on the color of the cylinder for identification because color-coding is not standardized and may vary with the manufacturer or supplier.
Transporting Cylinders
- Always use a hand truck equipped with a chain or belt for securing the cylinder.
- Make sure the protective cap covers the cylinder valve.
- Never transport a cylinder while a regulator is attached.
- Always use caution when transporting cylinders – cylinders are heavy.
- Avoid riding in elevators with compressed gas cylinders. If this is necessary, consider using a buddy system to have one person send the properly secured cylinders on the elevator, while the other person waits at the floor by the elevator doors where the cylinders will arrive.
- Do not move compressed gas cylinders by carrying, rolling, sliding, or dragging them across the floor.
- Do not transport oxygen and combustible gases at the same time.
- Do not drop cylinders or permit them to strike anything violently.
Safe Storage Procedures
- Gas cylinders must be secured to prevent them from falling over. Chains are recommended over clamp-plus-strap assemblies because straps can melt or burn in a fire. Be sure the chain is high enough (at least 2/3 up) on the cylinder to keep it from tipping over.
- Cylinders should be removed from the gas cylinder hand truck and secured appropriately before attaching a regulator.
- Do not store incompatible gases right next to each other. Cylinders of oxygen must be stored at least 20 linear feet away from cylinders of hydrogen or other flammable gas, or the storage areas must be separated by a firewall five feet high with a fire rating of 1/2 hour.
- All cylinders should be stored away from heat and away from areas where they might be subjected to mechanical damage.
- Keep cylinders away from locations where they might form part of an electrical circuit, such as next to electric power panels or electric wiring.
- The protective cap that comes with a cylinder of gas should always be left on the cylinder when it is not in use. The cap keeps the main cylinder valve from being damaged or broken.
Operation of Compressed Gas Cylinders
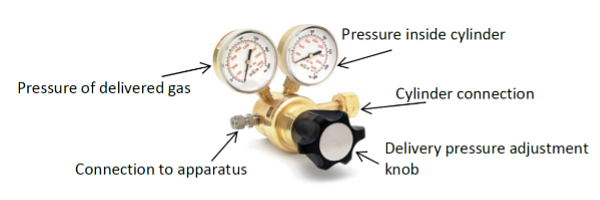
The cylinder valve hand wheel opens and closes the cylinder valve. The pressure relief valve is designed to keep a cylinder from exploding in case of fire or extreme temperature. Cylinders of very toxic gases do not have a pressure relief valve, but they are constructed with special safety features. The valve outlet connection is the joint used to attach the regulator. The pressure regulator is attached to the valve outlet connector to reduce the gas flow to a working level.
The Compressed Gas Association has intentionally made certain types of regulators incompatible with certain valve outlet connections to avoid accidental mixing of gases that react with each other. Gases should always be used with the appropriate regulator. Do not use adaptors with regulators.
The cylinder connection is a metal-to-metal pressure seal. Make sure the curved mating surfaces are clean before attaching a regulator to a cylinder. Do not use Teflon tape on the threaded parts, because this may cause the metal seal not to form properly. Always leak test the connection.
Basic Operating Guidelines
- Make sure the cylinder is secured.
- Don the appropriate PPE. Always use safety glasses, preferably a face shield and gloves.
- Attach the proper regulator to the cylinder. If the regulator does not fit, it may not be suitable for the gas you are using.
- Attach the appropriate hose connections to the flow control valve. Always secure any tubing with clamps so that it will not whip around when pressure is turned on. Use suitable materials for connections; toxic and corrosive gases require connections made of special materials.
- Install a trap between the regulator and the reaction mixture to avoid backflow into the cylinder.
- To prevent a surge of pressure, turn the delivery pressure adjusting screw counterclockwise until it turns freely and then close the flow control valve.
- Slowly open the cylinder valve hand wheel until the cylinder pressure gauge reads the cylinder pressure.
- With the flow control valve closed, connection to apparatus, turn the delivery pressure screw clockwise until the delivery pressure gauge reads the desired pressure.
- Adjust the gas flow to the system by using the flow control valve or another flow control device between the regulator and the apparatus.
- After an experiment is completed, turn the cylinder valve off first, and then allow gas to bleed from the regulator. When both gauges read “zero”, remove the regulator and replace the protective cap on the cylinder head.
- When the cylinder has at least 25 psi remaining, mark it as “Empty.” Store empty cylinders separate from full cylinders.
- Attach a “Full/In Use/Empty” tag to all cylinders. Tags are perforated and can be obtained from the gas cylinder vendor.
Precautions
- Use a regulator only with gas for which it is intended. The use of adaptors or homemade connectors has caused serious and even fatal accidents.
- Toxic gases should be purchased with a flow-limiting orifice.
- When using more than one gas, be sure to install one-way flow valves from each cylinder to prevent mixing. Accidental mixing can cause contamination of a cylinder.
- Do not attempt to put any gas into a commercial gas cylinder.
- Do not allow a cylinder to become completely empty. Leave at least 25 psi of residual gas to avoid contamination of the cylinder by reverse flow.
- Do not tamper with or use force on a cylinder valve.
Return of Cylinders
Disposal of cylinders and lecture bottles is expensive, especially if the contents are unknown. Make sure that all cylinders and lecture bottles are labeled and included in your chemical inventory.
Before you place an order for a cylinder or lecture bottle, determine if the manufacturer will take back the cylinder or lecture bottle when it becomes empty. If at all possible, only order from manufacturers who will accept cylinders or lecture bottles for return.
There is a limit, based on "Oregon Fire Code" of the total amount of hazardous gas that be present in each "control area" called Maximum Allowable Quantity (MAQ). The MAQ decreases for the floors higher in the building. Both empty and full gas cylinders are included when determining the MAQ. Please consult with EHS if you are considering purchasing hazardous gases.
